

SODIUM COCO SULFATE VS SODIUM LAURYL SULFATE:
WHAT’S THE DIFFERENCE?
Lots of personal care products proudly say they’re “SLS-free” and one way to go that way, is to use Sodium Coco Sulfate (SCS). As it turns out, SCS is not all that different to Sodium Lauryl Sulfate (SLS).
What was wrong with SLS in the first place?
Sodium Lauryl Sulfate is widely used in shower gels, shave gels, mouthwashes, soaps, shampoo’s and even toothpastes. This chemical looks like this:
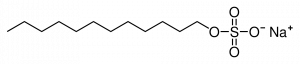
Structure of Sodium Lauryl Sulfate (SLS)
As with most detergents, these sulfates have an oil-soluble “tail” (seen on the left) and a water-soluble “head” (seen on the right). This gives it a double purpose, it equally loves water and oil which is its main use in personal care products.
When used with copious amounts of water, the Sulfates are fantastic at helping to separate oils from hair and skin – a little too good sadly. Those oils the Sulfates are trying to remove form an important part of your skin barrier, to keep things out and keep the water in.
If you remember from our “The Secrets Of Sulfates In Skincare” post, the very oil SLS is so good at stripping away, causes irritation. 5% SLS in a product is usually enough to cause irritation in most people, but many people are sensitive to far less, yet controversially it is used in the 15-50% concentration range, far exceeding the recommended safe levels.
So what’s the difference between SLS and SCS?
Turns out, SCS contains SLS; the difference lies in the science. The process to make SLS involves a chemical reaction that isolates one fatty acid from either petroleum, coconut oil or palm oil, Lauric Acid (C12).
Whereas SCS is derived from a blend of fatty acids from coconut oil, C12- C18. While we all know and love coconut oil, its science lab derivatives aren’t completely natural ingredients and strip away most of the beneficial components. From the typical fatty acid composition of coconut oil (Table 1: Approximate Fatty acid content of Coconut oil) we can see that sodium coco sulfate could be about 66% SLS! [50/(50+16+8+2)].
| Table 1: Approximate Fatty acid content of Coconut oil | ||||
|---|---|---|---|---|
| Type of fatty acid | % | |||
| Caprylic saturated C8 | 8% | |||
| Decanoic saturated C10 | 8% | |||
| Lauric saturated C12 | 50% | |||
| Myristic saturated C14 | 16% | |||
| Palmitic saturated C16 | 8% | |||
| Oleic monounsaturated C18:1 | 2% | |||
| Other | 8% | |||
| black: Saturated; grey: Monounsaturated; blue: Polyunsaturated | ||||
The proportion of SLS in SCS is not strictly defined nor regulated and manufacturers can make it as high as they like, all at the manufacturer’s sole discretion.
How does Sodium Coco Sulfate affect us?
Whether you use SLS or SCS you will have the exact same risks of skin irritation, eye irritation, acne causation, stripping hair of natural oils, premature skin ageing and forming nitrosamines in the presence of triethanolamine as SLS does. That’s because SLS forms a major part of SCS and like all Sulfates, all are Ionic detergents as we discuss further in this article.
How to be truly SLS free:
With the growing evidence on the damage caused by SLS and other Sulfates, many companies have switched to a more natural sounding chemical but be wary. They are playing the ‘Green Washing Game’ with you and your safety. It’s not unusual to see an ‘SLS Free’ claim made with a product that contains SCS or a similar Sulfate. The only way to be certain, is to look out for ‘Sulfate free’, like our entire skincare range.
We know how tough it can be to understand the gibberish that often disguised as an ingredient. In our mission to promote a healthy skin, lifestyle and world, we believe that the first step is advocating for educated consumption. We have a great article on how to decode the ingredients list:
The bottom line:
if Sulfates (and other ionic detergents) cause high rates of irritation in people and may have potential cancer-causing contaminants present, why are these detergents used rather than a safer, alternative?
It really boils down to 2 reasons.
- Consumer perception – When you think of cleaning something, you think of foam! Lots of foam. Those little bubbles magically lifting away dirt. On a psychological level people like to see something happening, but all you’re seeing is soap molecules trapping air in spherical pockets. Trapped air in pockets DOES NOT CLEAN. The molecules in detergents perform the cleaning action. Adding Ionic detergents, which are fantastic at creating lots of foam satisfy the mis-marketing around the notion it’s the foam which provides the cleansing action.
- Price – Ionic surfactants & especially chemicals such as SLS, SCS, SLES & ALS are far cheaper to manufacture. When you account for all the expenses a typical business has, it is far easier to make savings in the department most consumers do not see – the ingredients. It’s why we recommend to check the ingredients list on everything you buy. You can use our excellent guide published here.
We hope that this explanation clears up the confusion surrounding sulfates in skincare. My recommendation would be to not only avoid the usual suspects, but also other ionic detergents too.
Fortunately, there are several ionic-detergent-free products out there now—including all the entire R10 Labs Skincare range.
The science in skincare is our ongoing series helping consumers better understand the science in skincare. We translate the science into a format that is much easier to read, bust the myths and give you a clear, transparent and honest assessment so you can make an informed choice of what goes onto your skin.
Be the first to hear about our new articles by signing up to our email newsletters or by following us on Facebook or Twitter

 Are You Buying Skincare The Smart Way?
Are You Buying Skincare The Smart Way?



Sodium Coco Sulfate is made from coconut oil. The molecular weight of the resulting detergent is much larger than SLS and won’t penetrate the deeper skin levels like SLS does. SLS has a much smaller molecular size and so penetrates the skin causing irritation and yes with derivatives from petroleum too, nasty! Please don’t bash SCS so much, it’s not a green washed ingredient. Coconuts do contain fatty acids such as lauric acid that is used for SLS (hence lauryl/laureth) but the lauric acid isn’t a problem in SCS it contains lots of other fatty acids too. SCS is much milder and better on the environment too, no palm oil unlike SLS! Nice products by the way. :)
As I explained in the post, yes, SCS is made from Coconut oil & while we all know and love coconut oil, its science lab derivatives aren’t completely natural ingredients and strip away most, if not all, of the beneficial components.
As SCS contains ~66% of SLS due to coconut oils fatty acid content, SCS has the exact same problems and issues as SLS, not to mention both are ionic surfactants which denature protein-protein bonds, causing irritation by penetrating the skin. It’s only fair consumers know what they are purchasing. The science behind skincare is not easy to understand and we do try to break it down into an understandable format. It dismays me to see some companies shout loudly at being SLS free when they just use SCS or another ionic surfactant instead – whether it’s done through ignorance or deception, neither is acceptable.
The current issue around Palm oil isn’t because palm oil is inherently bad for the environment, it isn’t. It is the issue of weak governance and ineffective policing which allows unscrupulous palm oil producers to destroy vital habitats in the pursuit of profits. I really should do an article which explains this in depth! :)