The issue with Ionic Detergents.
All detergents, can be classified in one of three categories:
1. IONIC
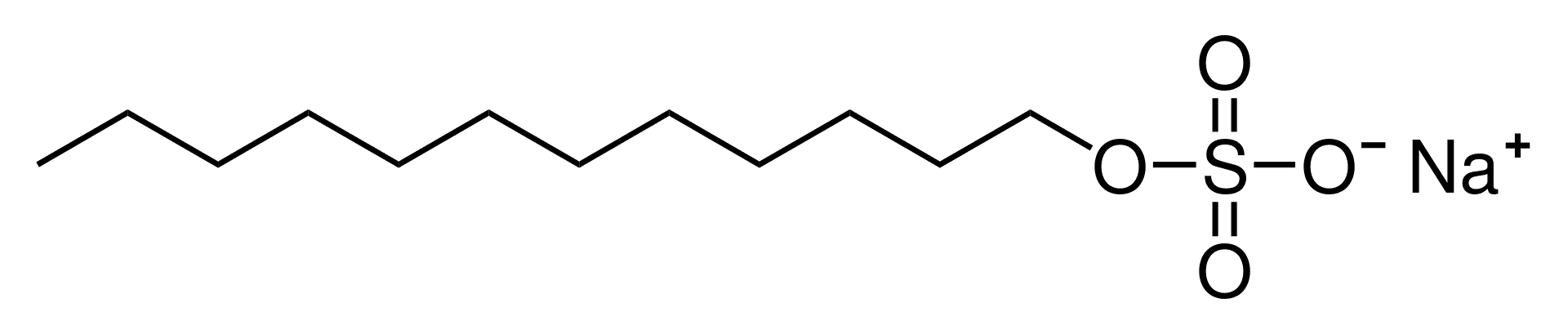
The polar head group of ionic detergents contain either a positive (cationic) or negative (anionic) charge.
Examples:
- Sodium Lauryl Sulfate (SLS)
- Sodium Laureth Sulfate SLES
- Sodium Coco-Sulfate
- Sodium Cocoyl Sarcosinate
- Sodium Lauroyl Isoethionate
- Sodium Lauroyl Taurate
- Disodium Laureth Sulfosuccinate
- Sodium Pareth Sulfate
- Sodium Lauroyl Sarcosinate
- Sodium Myreth Sulfate
Ionic detergents are considered biologically “harsh” detergents because they typically bind to & modify protein structures and for this purpose is often used in laboratories to disrupt cellular structures. Skin aging as a result of sun exposure is believed to occur because of cellular structure disruption, manifesting itself in the well-known skin burn. In other words, Ionic surfactants such as SLS, SLES & ALS has the ability to cause premature skin aging.
Studies have verified that this ingredient can be damaging. In the International Journal of Toxicology, researchers noted that it had a “degenerative effect on the cell membranes because of its protein denaturing properties,” and that it could cause skin irritation and corrosion. Researchers later wrote, “The longer these ingredients stay in contact with the skin, the greater the likelihood of irritation, which may or may not be evident to the user.”
They add in their discussion of the study that the ingredient was found to cause “severe epidermal changes” where it was applied, and that it could also damage the hair follicle (when used in hair-care products). Even worse —a solution containing 1.5% sodium lauryl Sulfate caused acne. The researchers wrote: “These two problems—possible hair loss and pimple formation—along with proven irritancy, should be considered in the formulation of cosmetic products.”
2. ZWITTER-IONIC
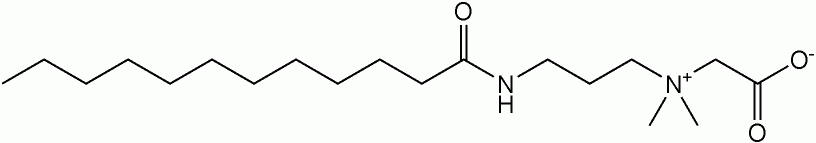
The polar head contain both negatively and positively charged atomic groups therefore it can have either a negative charge or a positive charge, depending on the pH.
Examples:
- Cocamidopropyl Betaine (CAPB)
- Sodium Cocoamphoacetate
- Sodium Cocoyl Isethionate
- Cocamidopropyl Hydroxysultaine
- Sodium Lauroamphoacetate
Zwitterionic detergents share the characteristics of both ionic and non-ionic detergents, with the strength of action of these compounds considered to be intermediate between ionic and non-ionic detergents and as such, zwitterionics are less denaturing than ionic detergents but more so than non-ionic detergents.
3. NON-IONIC
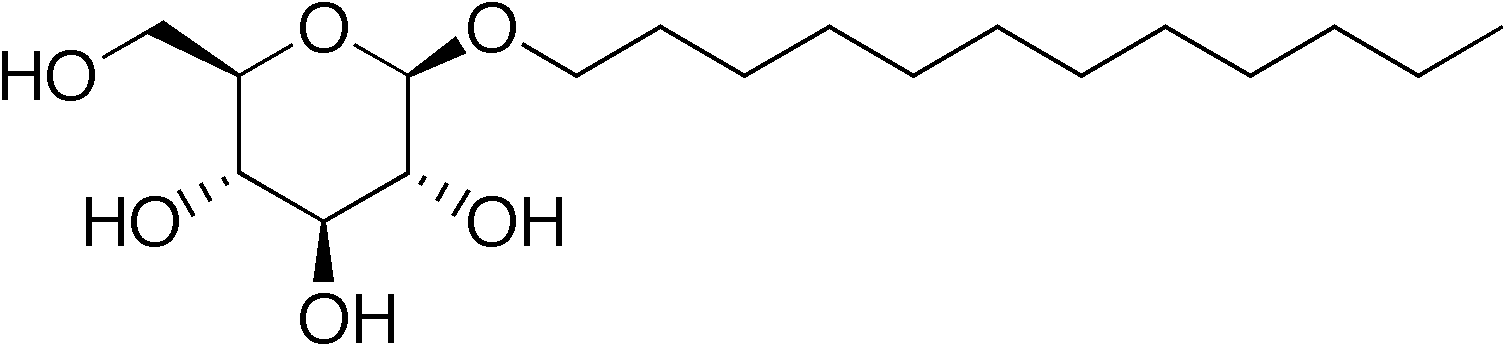
The polar head group of Non-ionic detergents contain molecules with head groups that are uncharged.
Examples:
- Coco Glucoside
- Decyl Glucoside
- Lauryl Glucoside
- Decyl Polyglucose
Non-ionic detergents are considered to be “mild” detergents because they are less likely than ionic detergents to denature proteins. By not separating protein-protein bonds, non-ionic detergents allow the protein to retain its native structure and functionality reducing the chance of irritation and premature skin ageing. (Our entire skincare range only uses non-ionic detergents for this reason).
So where does this leave you?
As is often noted in studies, SLS, SLES, ALS and other Ionic detergents can be somewhat safely used in concentrations below 1% and if thoroughly washed off. However most personal care products include Ionic surfactants in the 15-50% concentration range, far exceeding the recommended safe levels.
Then there is the issue of washing the products containing ionic detergents off thoroughly. If you’ve noticed after a shower, when you step out and smell your skin, there is a light fragrance remaining? Well that’s because you aren’t washing the product off completely – and it’s not your fault. Due to the intricate complexities of physics, biology and chemistry, it is practically impossible to remove every single trace of product you have applied.
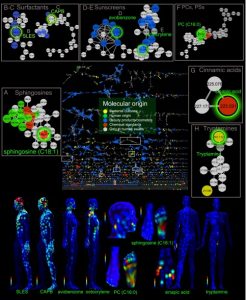
In a study published in the prestigious PNAS, a Molecular cartography of the human skin surface in 3D was taken. As seen by the image below, high quantities of SLES & CAPB, the two detergents tested for, was found in varying concentrations across the skin.
The bottom line:
if Sulfates (and other ionic detergents) cause high rates of irritation in people and may have potential cancer-causing contaminants present, why are these detergents used rather than a safer, alternative?
It really boils down to 2 reasons.
- Consumer perception – When you think of cleaning something, you think of foam! Lots of foam. Those little bubbles magically lifting away dirt. On a psychological level people like to see something happening, but all you’re seeing is soap molecules trapping air in spherical pockets. Trapped air in pockets DOES NOT CLEAN. The molecules in detergents perform the cleaning action. Adding Ionic detergents, which are fantastic at creating lots of foam satisfy the mis-marketing around the notion it’s the foam which provides the cleansing action.
- Price – Ionic surfactants & especially chemicals such as SLS, SLES & ALS are far cheaper to manufacture. When you account for all the expenses a typical business has, it is far easier to make savings in the department most consumers do not see – the ingredients. It’s why we recommend to check the ingredients list on everything you buy. You can use our excellent guide published here.
We hope that this explanation clears up the confusion surrounding sulfates in skincare. My recommendation would be to not only avoid the usual suspects, but also other ionic detergents too.
Fortunately, there are several ionic-detergent-free products out there now—including all the entire R10 Labs Skincare range.
(1) Quantification of sodium lauryl sulfate penetration into the skin and underlying tissue after topical application–pharmacological and toxicological implications. – Patil S, Singh P, Sarasour K, Maibach H. https://www.ncbi.nlm.nih.gov/pubmed/8801341
(2) 7 Final Report on the Safety Assessment of Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate http://journals.sagepub.com/doi/abs/10.3109/10915818309142005
(3) The determination of the irritancy potential of surfactants using various methods of assessment. https://www.ncbi.nlm.nih.gov/pubmed/755672
(4) “NICNAS Existing Chemicals Information Sheet,” Australian Government Department of Health and Aging, October 9, 2007. http://nicnas.gov.au/Publications/Information_Sheets/Existing_Chemical_Information_Sheets/ECIS_sls_PDF.pdf.
The science in skincare is our ongoing series helping consumers better understand the science in skincare. We translate the science into a format that is much easier to read, bust the myths and give you a clear, transparent and honest assessment so you can make an informed choice of what goes onto your skin.
Be the first to hear about our new articles by signing up to our email newsletters or by following us on Facebook or Twitter
 Are You Buying Skincare The Smart Way?
Are You Buying Skincare The Smart Way?



Leave A Comment